10月25日,来自哈佛大学化学与化学生物学系、HHMI以及Broad Institute的David Liu教授实验室在Nature杂志上以长文形式(Article)发表了题为“Programmable base editing of A•T to G•C in genomic DNA without DNA ”的突破性成果,报道了一种新型腺嘌呤碱基编辑器 (ABE),它可以将A•T碱基对转换成G•C碱基对,加之此前报道的将G•C碱基对转换成T•A 碱基对的成果,该技术首次实现了不依赖于DNA断裂而能够将DNA四种碱基A、T、G、C进行替换的新型基因编辑技术。
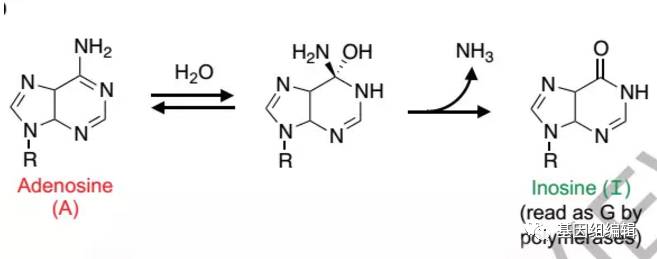
过去,单碱基突变的技术局限于只能将G•C碱基对转换成T•A 碱基对,对于将A•T碱基对转换成G•C碱基对一时还无法解决。这一主要原因就是没有找到合适的腺嘌呤脱氨酶。有研究表明,腺嘌呤脱氨酶一般只针对单碱基的腺苷或腺嘌呤或者错配的RNA:DNA异聚体,而不能在正常的DNA链上进行脱氨。
在这项研究中,David Liu实验室的研究人员通过使用蛋白进化和蛋白工程的手段通过大量的实验终于成功改造获得了能够对正常DNA上腺嘌呤脱氨的酶——E. coli TadA(ecTadA),然后将ecTadA与dCas9融合(ecTadA-dCas9)就可以进行检测了。
David Liu实验室的研究还表明,ecTadA-dCas9碱基编辑器对细菌细胞和人类细胞的DNA均有效。且在人类细胞中,它们能够在大范围的目标区域内引入预期遗传突变,效率约为50%,高于任何其它基因组编辑方法的效率,而且几乎无任何不良副作用,如随机插入、删除或其它突变等。
原文标题:
Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage
The spontaneous deamination of cytosine is a major source of C•G to T•A transitions, which account for half of known human pathogenic point mutations. The ability to efficiently convert target A•T base pairs to G•C could therefore advance the study and treatment of genetic diseases. While the deamination of adenine yields inosine, which is treated as guanine by polymerases, no enzymes are known to deaminate adenine in DNA. Here we report adenine base editors (ABEs) that mediate conversion of A•T to G•C in genomic DNA. We evolved a tRNA adenosine deaminase to operate on DNA when fused to a catalytically impaired CRISPR-Cas9. Extensive directed evolution and protein engineering resulted in seventhgeneration ABEs (e.g., ABE7.10), that convert target A•T to G•C base pairs efficiently (~50% in human cells) with very high product purity (typically ≥ 99.9%) and very low rates of indels (typically ≤ 0.1%). ABEs introduce point mutations more efficiently and cleanly than a current Cas9 nuclease-based method, induce less off-target genome modification than Cas9, and can install disease-correcting or disease-suppressing mutations in human cells. Together with our previous base editors, ABEs advance genome editing by enabling the direct, programmable introduction of all four transition mutations without double-stranded DNA cleavage.
特别声明:本文来源于"基因组编辑",仅仅是出于传播信息的需要,作者如果不希望被转载或者联系转载稿费等事宜,请与我们接洽。